Understanding neural plasticity requires understanding the effect of age on functional systems. As one of the main aims of the Cam-CAN project is to understand how functional networks are affected by age, a number of ongoing analysis focuses on developing methods for understanding functional responses.
Current investigations
Currently a number of methodological developments are underway for the Cam-CAN project for analysing both incoming fMRI and MEG data:
1. Multimodal analysis of simple sensorimotor responses
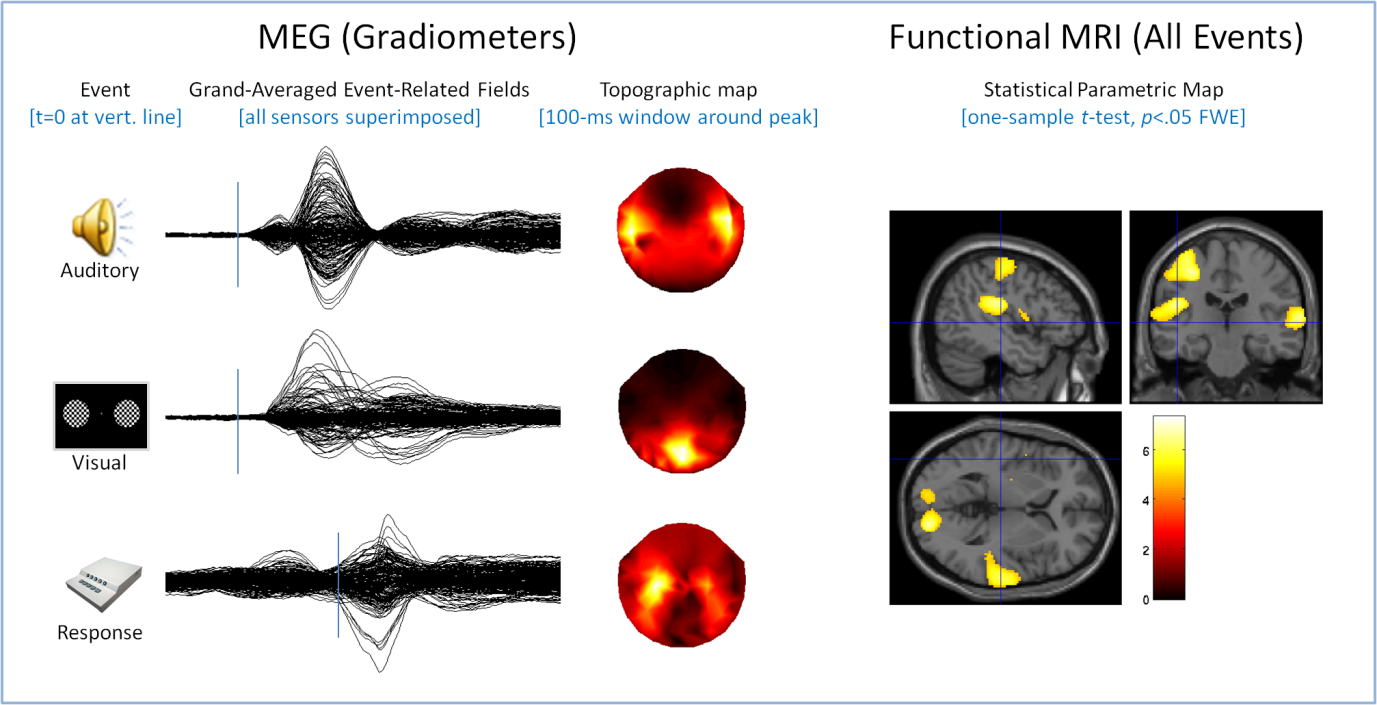
We are using a multimodal approach to characterise the neural responses to simple sensory (brief auditory tones, brief visual checkerboard) and motor (right-hand button press) events. Magnetoencephalography (MEG) data have a high temporal resolution(on the order of msec), which allows us to directly assess latency differences in these neural responses. Functional magnetic resonance imaging (fMRI) measures have high spatial resolution (on the order of mm), which allows us to pinpoint the location of activity associated with a sensorimotor event.
We are developing several analysis pathways that will integrate across the two modalities. For example, statistical maps of fMRI activations can be used to position dipoles for MEG source estimation. Estimates of MEG source amplitude can be used to estimate to what extent any age-related differences in fMRI blood oxygenation-level dependent (BOLD) contrast are due to changes in neuro-vascular coupling rather than to bona fide differences in neural responses. The figure shows some preliminary results from~200 subjects in each modality, MEG (left, separately for each event) and fMRI (for all events superimposed), consistent with the expected pattern of activation (bilateral auditory and visual cortex and left motor cortex).
2. MEG pre-processing tools
A major challenge for current and future MEG data is to speed the process of removing artefacts in such a large scale dataset. If the MEG sensor net can be considered as a sphere, then artefacts originating outside that sphere can be attenuated using an application called MaxFilter. We are developing methods to optimize the use of MaxFilter to eliminate artefacts and to employ MATLAB code to implement MaxFiltering for computationally efficient denoising of MEG datasets. To remove artefacts originating from inside the MEG sensor net, we used an ICA(Independent Component Analysis)-based denoising algorithm. We were able to select ICs (Independent Components) relating to ECG(Electrocardiogram), VEOG (Vertical Electro-oculogram) and HEOG(Horizontal Electro-oculogram) and remove them through a novel bootstrap-based thresholding scheme.
3. fMRI resting state analysis
Complex functional brain networks are networks of brain regions and interregional correlations, constructed from functional brain imaging datasets. The technological sophistication of modern functional brain imaging, such as functional magnetic resonance imaging (fMRI) and magnetoencephalography (MEG) allows the acquisition of increasingly accurate and high-resolution functional brain networks. A small number of statistical measures provide a remarkable amount of information about the local and global properties of these networks, as recently reviewed (Rubinov and Sporns, 2010; Bullmore and Bassett 2011). Recent years have seen much interest in the characterization of these networks in the resting state, or during a no-task condition.
Functional networks in the resting state have been shown to be heritable and reproducible, to change with administration of neuropharmacological agents, and to change in several neurological and psychiatric disorders, including Alzheimer’s disease and schizophrenia. In the Cam-CAN project, we aim to study the local and global properties of complex functional brain networks in healthy ageing.
4. MEG resting state analysis
Highly reproducible Resting-State Networks (RSNs) have been found in fMRI data, but very little work has been done on identifying RSNs in MEG data. Since MEG has high temporal resolution, it allows for identifying RSNs at higher frequency bands, which would not be visible in fMRI data. We propose to identify MEG resting-state networks in each of the cohort of 700, and investigate the relationship between some network features (eg. mean degree, clustering coefficient, efficiency) and age.
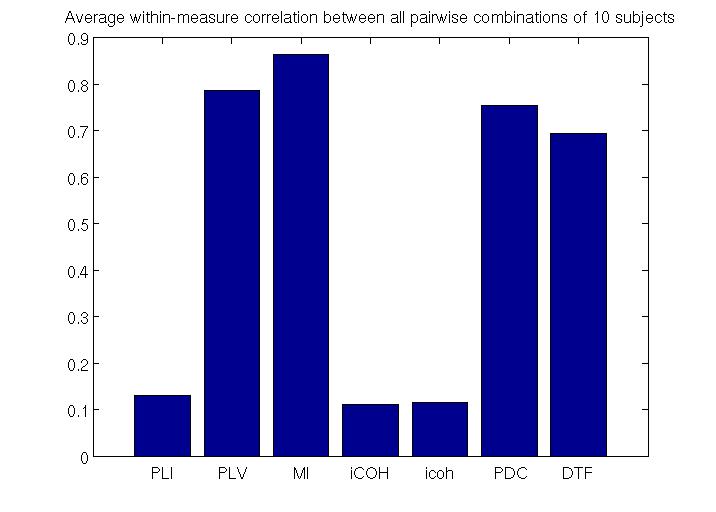
To estimate RSNs, we first need to choose a measure of connectivity. We evaluated the performance of different measures of effective (PDC, DTF) and functional (PLI,PLV,MI, iCOH, icoh)connectivity by comparing reproducibility of RSNs across 10randomly chosen subjects. Our current analysis suggests that PDC(Partial Directed Coherence) is the most robust measure of effective connectivity and MI (Mutual Information) is the most robust measure of functional connectivity (see figure below).
We are currently developing MATLAB code to calculate analytically defined thresholds, to distinguish PDC values and MI values which are statistically significant from those that are not. After this, we will be estimating MEG resting-state networks with PDC and MI, for different frequency bands, for each of the cohort of 700. Then, network features for each subject will be calculated and their relationships to age will be examined. Finally, we will be verifying if the low-frequency MEG resting-state networks translated to 3-D source space are related to the fMRI resting-state networks, while the high-frequency MEG resting-state networks are independent of the fMRI resting-state networks.

 Cambridge Centre for Ageing and Neuroscience
Cambridge Centre for Ageing and Neuroscience